Diabetes and Bone Health and Arthritis Treatment
Negative effects on bone health are caused by diabetes or
excessive blood sugar. Diabetes can undoubtedly be made more difficult by bone
and joint conditions including osteoporosis and Charcot joints. Naturally,
persons without diabetes can also get osteoporosis, particularly the elderly
and postmenopausal women. However, the activity of osteoclasts is increased in
diabetic patients due to metabolic problems. Osteoporosis manifests itself
early and severely due to impaired osteoblast activity.
Checking blood calcium, blood phosphorus, and alkaline
phosphatase may not reveal any issues for osteoporosis patients, but performing
an x-ray or a bone density test will reveal issues.
Osteoporosis can result in bone discomfort, fractures, and,
more significantly, incapacity.
To combat osteoporosis, early calcium and vitamin D intake is
essential. Charcot joints typically develop in the ankles and foot.
Skeletal abnormalities, particularly in severe and persistent
bone joints such ankle swelling after modest trauma, are the predominant
symptoms, and pain is less visible.
The exact process is unclear, although it may be linked to
diabetic vascular and neuropathy in this bone condition.
Treatment for Charcot joints consists primarily of local
care, such as avoiding heavy weight bearing on the affected limb or wearing
protective shoes. Joint fixation surgery may also be performed if necessary.
Osteoporosis and Diabetes
One of the major illnesses of the endocrine and metabolic
systems is osteoporosis, which is closely related to diabetes.
Each has a very convoluted aetiology, and when diabetes and
osteoporosis coexist in one person, the situation becomes even more
challenging.
A brief summary of the relationship between diabetes and
osteoporosis is provided below:
1. Changes in bone mineral density and the frequency of fragility fractures in diabetes patients
It is undeniable that people with diabetes are more likely to
have fractures. Patients with type 1 diabetes are more likely to have
osteopenia and osteoporosis, with rates ranging from 48% to 72%.
Do diabetics face an increased risk of fracture?
Compared to people without diabetes and people with adequate
glycemic control, patients with type 2 diabetes who had poor glycemic control
had a 47%–62% increased risk of fracture. Femoral neck and spine fracture
chances were, respectively, 2.1 and 3.1 times greater than those of the general
population.
Are diabetics more likely to suffer a hip fracture?
Patients with both type 1 and type 2 diabetes had a
noticeably increased risk of hip fracture than healthy people, according to a
substantial meta-analysis.
Thus, compared to the general population, the frequency of
osteoporosis and the risk of osteoporotic fractures are much higher in the
overall diabetic community.
However, the issue becomes more problematic when considering bone density in relation to the prevalence of osteoporosis in the diabetic population.
Do people with diabetes have an imbalance between bone resorption and formation?
A balance between bone creation and bone resorption results from the fact that two-thirds of type 1 diabetic patients are in a state of bone turnover with a prevalence of bone resorption. This explains the elevated risk of fractures brought on by lower bone density, which is connected to bone loss in type 1 diabetes and absolute insulin shortage. a reduction in matrix synthesis
It is still debatable, nevertheless, how type 2 diabetes patients' bone mineral density varies.
Do people with diabetes experience a decline in bone mineral density?
Numerous investigations have discovered that the increased risk of fracture in people with type 2 diabetes does not always coincide with a loss of bone mineral density.
In fact, some research results even imply that the level of bone mineral density is higher than that of healthy controls. This is primarily related to the loss of bone mass that occurs in type 2 diabetes, and the factors that affect bone mass in type 2 diabetes are more complex than they are in type 1 diabetes. These factors include body mass index (BMI), insulin resistance, protein glycation, an increased risk of falls, and the use of some oral hypoglycemic medications, among others.
Are persons with type 2 diabetes considerably more likely to have higher BMDs?
Further investigation revealed that while type 2 diabetes
patients' BMD was much higher than that of the control group, the increase was
primarily focused in the trabecular bone, with no change in the cortex's bone
composition. Dual-energy x-ray absorptiometry (DXA), according to some experts,
is difficult to detect the minor changes in bone structure and bone quality in
individuals with type 2 diabetes, leading to an elevated fracture risk in these
patients that is independent of their bone density. Pproper monitoring and
evaluation serve as an example of this.
Is the cortical bone's quality compromised in those with type 2 diabetes?
Another study indicated that patients with type 2 diabetes
had higher trabecular bone mineral density of the distal tibia and radius and
higher radial cortex porosity, indicating that the quality of the cortical bone
in these patients was compromised, impacting the risk of diabetic fractures.
2. Bone turnover in diabetic patients
Bone turnover status is an important part of the pathogenesis of osteoporosis. Classification by bone turnover rate Osteoporosis is divided into three types:
- High turnover
- Normal turnover
- Low turnover
For instance, the high turnover type includes postmenopausal
osteoporosis, hyperparathyroidism, and glucocorticoid-induced osteoporosis.
The term "bone turnover" in medical terminology
refers to the processes of osteoblasts, which produce new bone, and
osteoclasts, which break down existing bone.
Numerous bone turnover biomarkers, including osteocalcin,
bone-specific alkaline phosphatase (BAP), osteoprotegerin (OPC), type 1
procollagen amino-terminal peptide (PINP), etc., are secreted during the
process of bone turnover. In contrast, type I collagen N-terminal peptide (NTX)
and type 1 collagen C-terminal peptide (CTX), which are both associated with
bone resorption, are bone formation indicators
The status of bone metabolism can be reflected more swiftly and sensitively by changes in bone turnover index, which changes more quickly than changes in bone density.
For the diagnosis of high-transformation osteoporosis and the tracking of treatment effectiveness, it has significant clinical value. After receiving anti-bone resorption medication, such as alendronate, the bone resorption index will begin to significantly decline 2-4 weeks later and peak 3-6 months later.
The plateau was reached in between six and twelve months,
with the shift in the bone resorption index occurring a little later.
What about the rate of bone turnover in diabetics?
According to studies, osteocalcin levels fall by over 4 times
in people with type 1 diabetes, and they are inversely connected with HhA1c.
Do people with diabetes have more brittle bones?
Patients with type 1 diabetes who have less-than-ideal blood sugar control have more visibly fragile bones, which raises the possibility that hyperglycemia may harm bone growth.
Patients with type 2 diabetes also showed significant
declines in osteocalcin and sclerostin, and anomalies in these biomarkers
suggest that both type 1 and type 2 diabetics have limited bone turnover, which
leads to bone mineral loss.
3. The potential cause of osteoporosis brought on by diabetes
The difficult and incompletely understood process of preventing diabetes-related osteoporosis. It also has something to do with bone metabolism regulators and bone mineral metabolism in addition to gender, age, weight, race, and nutritional status, etc.
Diabetes can alter the metabolism of bones in a number of ways.
According to current thinking, the following elements play a role in osteoporosis development in people with diabetes:
1. The impact of high blood sugar on osteoblasts
According to research, high glucose levels (12 mmol/L or even 24 mmol/L) can affect osteoblasts' biomineralization processes and promote mineralization by elevating RANKL, bone sialoprotein, and transcription.
Reduced OPG mRNA expression caused by the receptor Runx2
lowers the grade of the minerals.
TLR-2, -3, -4, and -9 are overexpressed in osteoblasts as a
result of the high osmotic pressure environment brought on by high glucose
levels, which affects the differentiation, maturation, and function regulation
of osteoblasts and osteoclasts.
The aforementioned high-glucose environment exerts a number of impacts on osteoblasts that ultimately cause the level of serum osteocalcin, which is essential for bone mineralization, to decline.
Recent research has revealed that osteocalcin is a separate
component that affects HbA1c and is closely linked to the escalation of
problems of glucose metabolism. Reduced osteoblast activity may be reflected in
diabetic patients' lower levels of osteocalcin.
Osteoblasts respond differently to varied glucose
concentrations. The proliferation of MG63 osteoblasts was first encouraged by
gradually raising the glucose concentration, and later it was suppressed.
The effect of glucose on causing apoptosis of MC3T3-E1
osteoblasts became more apparent when the glucose concentration increased from
11.1 to 33.3mmol/L, and with the extension of culture period, the apoptosis of
MC3T3-E1 osteoblasts also increased dramatically.
According to its ability to cause osteoblast apoptosis, which is concentration- and time-dependent, a high glucose environment may be harmful to osteoblasts.
High glucose levels reduced osteoblast development and
maturation in addition to enhancing osteoblast apoptosis.
According to preliminary study, hyperglycemia reduces BMSCs' ability to differentiate into osteoblasts in a dose-dependent way.
In addition to directly affecting osteoblast differentiation and death, excessive glucose levels also indirectly influence osteoblast activity via controlling PPARy expression.
PPARy is a crucial transcription factor for adipokines and a member of the nuclear receptor transcription factor superfamily.
Chronic long-term hyperglycemia upregulates PPAHy expression,
which inhibits osteoblast growth.
2. How hyperglycemia affects osteoclasts
Through the control and differentiation of osteoblasts by RAHKL, OPG, and other factors, osteoclasts are produced from hematopoietic stem cells.
Osteoclasts can be stimulated by glucose, which is their main energy source. According to studies, glucose concentrations between 7 and 25 mmol/L can sustain the highest levels of bone resorption activity.
It is clear that the osteoclasts' ability to resorb bone is
glucose concentration-dependent, and hyperglycemia causes a fast bone loss.
3. How bone metabolism is affected by insulin-like growth factor 1 (IGF-I)
IGF-I can accelerate cell division, stimulate DNA synthesis in osteogenic cells, boost osteoblast differentiation and activity, and regulate bone resorption all at once.
It also inhibits the breakdown of collagen, a crucial growth factor released by skeletal cells.
IGF-I is now well known for promoting the growth of cells that resemble osteoblasts.
IGF-osteogenic I's function can be diminished by long-term
hyperglycemia in diabetic individuals by preventing the production and release
of IGF-I.
4. Insulin's impact on bone metabolism
Insulin can facilitate the formation of bone collagen tissue by acting on insulin receptors on the surface of bone cells.
Diarrhea-induced insulin deficit or insulin resistance can cause osteoblasts to malfunction, reduce the amount of bone matrix, and have an impact on osteocalcin synthesis.
Due to the absolute insulin shortfall in type 1 diabetes, the relative insulin insufficiency impacts the manufacture of collagen by osteoblasts, which can speed up the metabolism of collagen tissue and consequently increase osteoclasts' ability to resorb bone. At the same time, a shortage of insulin prevents osteoblasts from synthesising bone. Calcium causes bone resorption to outpace bone production, which eventually results in osteoporosis.
Insulin therapy is therefore advantageous for both treating and avoiding long-term diabetes problems, as well as for preventing osteoporosis.
The status of insulin cannot be disregarded if osteoporosis
is viewed as a chronic consequence of diabetes.
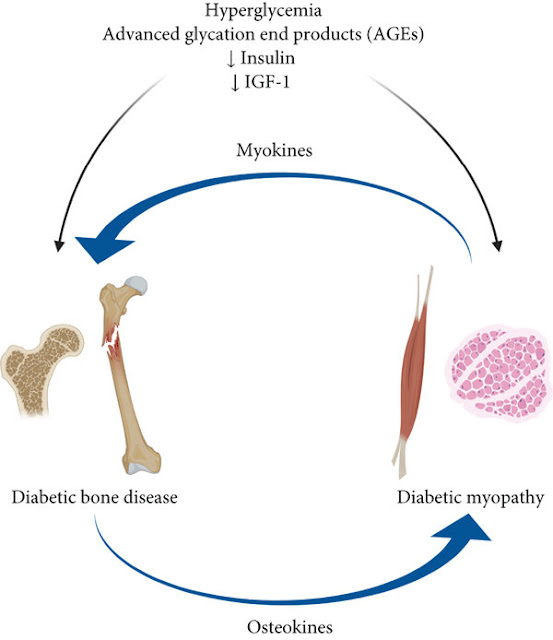
5. The impact of bones and advanced glycation end products (AGEs)
High glucose levels cause the production of numerous AGEs in
various organs and tissues, including bone matrix, and a significant buildup of
ACEs in bone tissue, which leads to the death of mesenchymal stem cells and a
reduction in osteogenesis by preventing their differentiation into adipose
tissue, cartilage, and bone.
Two processes of bone remodelling, namely bone resorption by osteoclasts and bone creation by osteoblasts, are impacted in diabetic patients by the glycosylation of bone proteins.
Additionally, through the osteoclast and osteoblast nuclear factor pathways, AGEs work with their receptors to encourage the adherence of numerous inflammatory factors such as interleukin (IL)-1, IL-6, TNF, intercellular adhesion molecules, and vascular cells.
Increased expression of molecule 1 alters the physiological
function of bone collagen, encourages osteoclast precursor maturation,
stimulates osteoclast aggregation, prevents osteoclast apoptosis, boosts
osteoclast activity, and speeds up bone resorption. These effects in turn cause
disorders of bone remodelling, which are a major factor in the development of
osteoporosis.
6. The effects of bone problems caused by diabetes
If long-term glucose control is inadequate, the great majority of diabetic patients will experience diabetic vascular problems, which also negatively affect bone metabolism.
In addition to increased bone loss, hyperparathyroidism brought on by diabetic nephropathy can also cause greater mobilisation of calcium from the bones.
Combining peripheral vascular disease with microcirculation issues causes the basement membrane to thicken around the capillaries, which affects bone reconstruction. It also affects the distribution of blood vessels in the bones, which results in insufficient blood flow to the bone tissue, which leads to abnormal bone metabolism.
After a diabetic cerebral infarction, the affected limb's muscle strength and ability to maintain balance both deteriorate, increasing the risk of falling.
What impact do hypoglycemic medications have on osteoporosis?
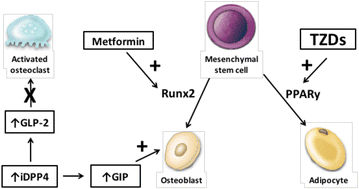
Thiazolidinediones (TZDs) were linked to increased bone loss and fracture risk in diabetic patients, particularly in women with diabetes, according to the ADOPT trial.
According to clinical data, TZD users who were aged postmenopausal women lost bone mass at a rate of 0.61% year compared to non-users, which was accompanied by a drop in blood osteocalcin levels.
At 8 weeks, rosiglitazone-treated mice displayed significant
decreases in bone mass, bone mineral density, and alterations in bone
microarchitecture.
These findings imply that TZDs have an impact on bone development
in type 2 diabetic patients. Studies have revealed that TZDs can encourage the
emergence and growth of osteoclasts. In addition, they can prevent mesenchymal
stem cells from differentiating into osteoblasts, which eventually causes
osteoporosis, and instead encourage the differentiation of mesenchymal stem
cells into adipocytes.
The most popular sulfonylureas in Asia also have an impact on diabetes patients' bone mass. According to UK-DPRD data, sulfonylurea-using individuals have a higher incidence of fractures.
These medications may hinder the phosphatase catalyst's ability to degrade by raising cAMP levels, inhibiting the enzyme's activity through competitive inhibition, and causing an increase in calcium salt loss.
Sulfonylurea medications have been found in recent domestic
studies to decrease the survival rate of MC3T3 E1 cells and increase the
expression of autophagy and apoptosis marker proteins, suggesting that medium
and high concentrations of sulfonylurea medications can induce autophagy and
apoptosis in osteoblasts. decreased ability of osteoblast differentiation.
4. Rehabilitation and Defense
First and foremost, it is important to achieve and maintain good blood sugar control, pay attention to the importance and status of insulin therapy, and use oral hypoglycemic medications carefully in diabetic patients with osteoporosis risk factors in order to prevent or delay the development of diabetes complications.
Without osteoporosis, diabetic people should be careful to
take calcium and vitamin D supplements while managing their diabetes to avoid
the onset and progression of osteoporosis.
Diabetes' Effect on Bone Disease
Even when a patient has had diabetes for more than ten years,
her blood sugar can still be managed.
She was found to have "several gallbladder stones"
following a recent checkup.
Can diabetes induce stones, the patient wonders?
Is this just an accident? Or are there further issues?
It's true:
Most osteoporosis sufferers experience a delayed onset that
lasts for several years.
Back and leg pain, twitching fingers, spasm of the
gastrocnemius muscle, brittle and easily broken bones, and even abnormalities
like stooping at the waist and lameness are the prominent clinical signs.
A bone density decline was seen on the X-ray. Bone issues can result in aberrant cartilage issues, which can cause osteoarthritis and limit an elderly person's ability to exercise.
The body's capacity to recuperate from exercise will also deteriorate, which will have an impact on the body's general health.
As is common knowledge, a category of metabolic illnesses
known as diabetes mellitus are characterised by persistently increased blood
sugar levels.
Low bone mass and microstructural bone tissue degradation are the hallmarks of the metabolic bone disease known as osteoporosis, which increases bone fragility and fracture susceptibility.
How can diabetes mellitus and osteoporosis, two conditions that seem incompatible, coexist?
The following factors can be used to determine the answer:
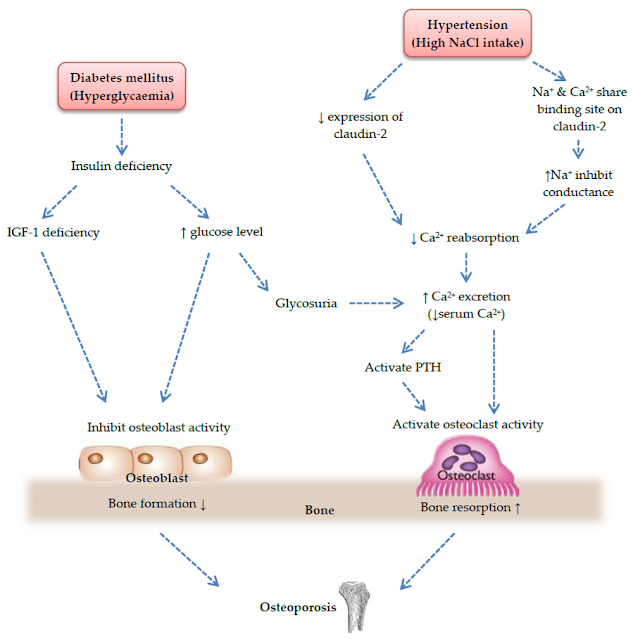
1. Diabetic patients' bodies always have high blood sugar
levels, which wrecks the body's calcium and phosphorus metabolism's delicate
equilibrium.
Patients with diabetes must excrete a lot of glucose through
the osmotic diuretic mechanism, and at the same time, a lot of minerals like
calcium, phosphorus, and magnesium must also be removed from the body. This
causes low levels of calcium and magnesium in the body.
As a result of this condition, the body will produce more
parathyroid hormone, which will exacerbate the osteolytic action and cause the
bone to "erode" and develop holes, leading to osteoporosis.
2. Osteoblasts, often known as "bone builders," are
in charge of bone rebuilding in human bones. Through insulin receptors on their
surface, osteoblasts' "bone-building" function is controlled by
insulin.
The "bone forming" effect of osteoblasts is diminished in diabetic individuals due to an absolute or relative shortage of insulin, which makes the human body more susceptible to osteoporosis.
3. Vitamin D is a crucial vitamin for enhancing calcium
activation and absorption. Vitamin D normally requires hydroxylase in renal
tissue to "activate." However, chronic diabetes damages the kidneys,
which makes it difficult for vitamin D to properly activate and greatly reduces
the activity of this hydroxylase in renal tissue. Because of a vitamin D
deficiency, calcium absorption is decreased.
4. Hypogonadism complicates the condition in a significant number of diabetic patients, and the absence of sex hormones itself will exacerbate and worsen osteoporosis (the process of osteoporosis in women is accelerated after menopause).
5. The nutritional deficit of bone metabolism will worsen
when diabetes mellitus is associated with vascular and neuropathy of bone
tissue.
Additionally, diabetes will worsen the situation for those
who already have senile osteoporosis and primary postmenopausal osteoporosis.
Osteoporosis is typically accepted as a natural part of
ageing, thus most people will accept it without seeking active therapy.
In actuality, osteoporosis is not only a normal physiological
ageing process; rather, it is a disease that, in the majority of instances,
requires medical attention and increases the risk of fracture to varying
degrees.
Statistics show that more than 50% of senior patients who
survive hip osteoporotic fractures are nevertheless crippled and unable to care
for themselves, and that over 20% of these patients pass away from various
complications within a year.
A third or less of diabetic patients can be diagnosed with
osteoporosis, and approximately half to two thirds of diabetic people have
decreased bone mineral density. Additionally, the human body's metabolism of
minerals is significantly influenced by bone health.
Osteoporosis can lead to cardiovascular disorders, which in turn influence the management of diabetes, as well as neurological and endocrine diseases and an imbalance in the body's mineral metabolism.
Because of this, it is crucial for diabetic patients to actively prevent the osteoporosis that is a side effect of diabetes mellitus.
Enhancing diabetic patients' quality of life while reducing medical costs is extremely important.
Patients with diabetes ought to value it very highly.
Simple diabetic treatment that lowers blood sugar for
stronger bones is quite doable.
How is diabetes treated?
Treatment options for diabetes include:
1. Changes in lifestyle, such as those related to nutrition, exercise, and blood sugar monitoring.
2. The use of medications such as metformin, glyburide, and others
3. Surgical procedures, such as diabetic insulin replacement therapy
4. Insulin combination therapy
5. Medication
6. Surgical procedure
7. Islet function reconstruction
How can diabetes be treated, and how can diabetes be treated for strong bones? blood sugar levels in just one week?
Diabetes treatment options
Treatment options for diabetes include:
1. Changes in lifestyle, such as those related to nutrition, exercise, and blood sugar monitoring.
2. The use of medications such as metformin, glyburide, and others
3. Surgical procedures, such as diabetic insulin replacement therapy
4. Insulin combination therapy
5. Drug
6. Surgical procedure
7. Islet function reconstruction
 |
| Dr. Shawna Reason |
Education: MBBS, MD
Occupation: Medical Doctor / Virologist
Specialization: Medical Science, Micro Biology / Virology, Natural Treatment
Experience: 15 Years as a Medical Practitioner
About Me | Linkedin | Quora Profile | Medium Profile | Twitter



Comments
Post a Comment